Cationic polythiophene–anionic fullerene pair in water and water–dioxane: studies on hydrogen bonding capabilities, kinetic and thermodynamic properties†
Abstract
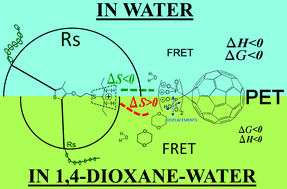
Despite the vast array of solution- and solid-state bio-analytical, bioelectronic and optoelectronic applications of cationic polythiophenes (CPTs), the number of studies focused on the role of hydrogen bonding (H-bonding) between these and other molecules is scarce, regardless of whether H-bonding is expected to play an important role in several such applications. Also, despite the advantages of using cosolvents to systematically examine the molecular interactions, there are no such studies for CPTs to our knowledge. This work presents a steady-state UV-vis/fluorescence spectroscopic, kinetic and thermodynamic study on the H-bonding interactions between a water-soluble, cationic–anionic (isothiouronium–tetraphosphonate), polythiophene–fullerene donor–acceptor pair with two-point, charge-assisted H-bonding (CAHB) capabilities, tuned using water or a 1,4-dioxane–water mixture (W–DI). Both solvents generate photoinduced electron transfer (PET), fluorescence resonance energy transfer (FRET), spontaneous binding, H-bonding, ground-state complexing via multiple site binding, formation of micelle-like aggregates and equivalence points at a similar concentration of the quencher. However, in comparison with water, W–DI promotes less-ordered, less packed micellar aggregates, due to hydrophobic desolvation of the H-bond and larger solvent displacement during the PT1–4Fo complexation. This would decrease the extent of charge-transfer and the size of the sphere-of-quenching, mainly by displacements or rotations of the H-bonds, instead of elongations, together with a possible larger extent of diffusion-controlled static quenching. At [4Fo] larger than the equivalence point the micelles formed in water do not have available binding sites due to a tighter aggregation, causing a decrease in the quenching efficiency, while the micelles formed in W–DI start showing larger quenching efficiencies, possibly due to an increase in entropy that overcomes the desolvation of the H-bonding. These results could be useful when analyzing outputs from systems including CPTs with H-bonding capabilities, operating in (or casted from) solvents with clear differences in polarity and/or H-bonding capacity.


 Please wait while we load your content...
Please wait while we load your content...