Two different regimes in alcohol-induced coil–helix transition: effects of 2,2,2-trifluoroethanol on proteins being either independent of or enhanced by solvent structural fluctuations†
Abstract
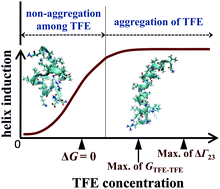
Inhomogeneous distribution of constituent molecules in a mixed solvent has been known to give remarkable effects on the solute, e.g., conformational changes of biomolecules in an alcohol–water mixture. We investigated the general effects of 2,2,2-trifluoroethanol (TFE) on proteins/peptides in a mixture of water and TFE using melittin as a model protein. Fluctuations and Kirkwood–Buff integrals (KBIs) in the TFE–H2O mixture, quantitative descriptions of inhomogeneity, were determined by small-angle X-ray scattering investigation and compared with those in the aqueous solutions of other alcohols. The concentration fluctuation for the mixtures ranks as methanol < ethanol ≪ TFE < tert-butanol < 1-propanol, indicating that the inhomogeneity of molecular distribution in the TFE–H2O mixture is unexpectedly comparable to those in the series of mono-ols. On the basis of the concentration dependence of KBIs between the TFE molecules, it was found that a strong attraction between the TFE molecules is not necessarily important to induce helix conformation, which is inconsistent with the previously proposed mechanism. To address this issue, by combining the KBIs and the helix contents reported by the experimental spectroscopic studies, we quantitatively evaluated the change in the preferential binding parameter of TFE to melittin attributed to the coil–helix transition. As a result, we found two different regimes on TFE-induced helix formation. In the dilute concentration region of TFE below ∼2 M, where the TFE molecules are not aggregated among themselves, the excess preferential binding of TFE to the helix occurs due to the direct interaction between them, namely independent of the solvent fluctuation. In the higher concentration region above ∼2 M, in addition to the former effect, the excess preferential binding is significantly enhanced by the solvent fluctuation. This scheme should be held as general cosolvent effects of TFE on proteins/peptides.


 Please wait while we load your content...
Please wait while we load your content...