Double substitution leads to a highly polymorphic system in 5-methyl-2-m-tolylamino-benzoic acid†
Abstract
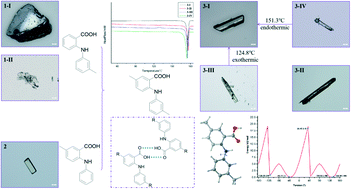
Addition of methyl group(s) to either or both aromatic rings of fenamic acid (FA) afforded three FA derivatives (1–3), designed to investigate the effect on the polymorphic behavior of these compounds exerted by substitution. A relatively comprehensive polymorph screen led to the discovery of four polymorphs for compound 3, for which substitution took place on both aromatic rings, in contrast to the production of either two or one form(s) for compounds 1 and 2, both of which are mono-substituted. The observation indicated that both substitution position and pattern are important in the polymorphism of these compounds. The thermal properties of each system were investigated by differential scanning calorimetry (DSC). Conformational scans and Hirshfeld surface analyses were performed to study the mechanism of polymorphism and the intermolecular interactions contributing to the stability of each crystal form.


 Please wait while we load your content...
Please wait while we load your content...