Stable hydrogen-bonded organic frameworks for selective fluorescence detection of Al3+ and Fe3+ ions†
Abstract
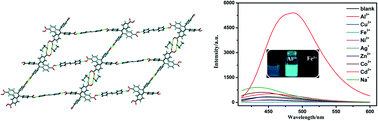
Four new hydrogen-bonded organic frameworks (HOFs) with the formulas {[H4TCPE·bpa]·(CH3OH·H2O)}n (1), {[H4TCPE·bpe]·(CH3OH·0.56H2O)}n (2), {[H4TCPE·bpa]·CH3OH}n (3) and {[H4TCPE·bpe]·(0.41CH3OH·0.59CH3CN)}n (4) (H4TCPE = tetrakis(4-carboxyphenyl)ethylene, bpa = 1,2-bis(4-pyridyl)ethane, bpe = 1,2-bis(4-pyridyl)ethylene) have been prepared under different conditions and structurally characterized. X-ray crystallographic analysis reveals that the hydrogen bond strengths of 3 and 4 synthesized by solvothermal method are much stronger than those of 1 and 2 prepared by the volatilization method. An obvious fluorescence shift was observed during the sensing process of 3 and 4 toward Al3+ and Fe3+ ions. For HOF 3, a red-shift emission (ca. 28 nm) occurs upon the addition of Al3+ ion. When Al3+ ion is added to HOF 4 in acetonitrile suspension, a red-shift emission (ca. 30 nm) as well as a great fluorescence enhancement happens. Both 3 and 4 exhibit strong fluorescence quenching with high selectivity and sensitivity toward Fe3+ ion. In addition, they display relatively good stabilities as well as reusability. Therefore, HOFs 3 and 4 are excellent fluorescent sensors toward Al3+ and Fe3+ ions.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...