Strong intermolecular interaction induced methylene-bridged asymmetric heterocyclic explosives†
Abstract
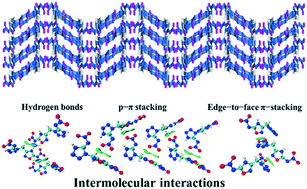
The design and synthesis of novel energetic materials with high energy and acceptable sensitivity is significant for the military and civil fields. In this study, a methylene-bridged asymmetric heterocyclic explosive based on strong intermolecular interactions, namely N-(1-((5-(nitroimine)-1,2,4-oxadiazol-3-yl) methyl)-1H-tetrazol-5-yl)nitroimine (4), was designed and synthesized. Its ammonium salt (5) and potassium coordination polymer (6) were also synthesized. The structures of compounds 4, 5 and 6 were determined by single crystal X-ray diffraction analyses. Independent gradient model (IGM) analyses and Hirshfeld surfaces (HS) analyses combined with single crystal analyses revealed the existence of intermolecular interactions in the three compounds. The strong intermolecular hydrogen-bonding, π⋯π, and p⋯π interactions enhance the molecular packing efficiency of 4, leading to high density and acceptable sensitivity. Detonation properties calculated with the professional software package EXPLO5 showed that the new metal-free explosive 4 exhibits superior detonation performance (D = 9296.8 m s−1; P = 38.3 GPa) which is comparable to that of HMX.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...