One-step solvothermal synthesis and growth mechanism of well-crystallized β-Ga2O3 nanoparticles in isopropanol
Abstract
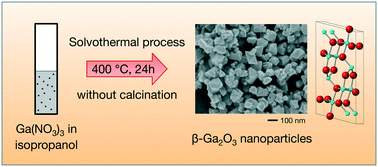
Simple liquid-phase approaches for the synthesis of nanomaterials are attractive because their low costs, reduced nanoparticle aggregation, and compatibility with subsequent liquid processes widen the application scope of the resulting materials. This would be particularly interesting for β-Ga2O3 nanoparticles, which often suffer from aggregation issues during their synthesis processes. In this paper, we report a one-step synthesis of β-Ga2O3 nanoparticles in supercritical isopropanol. By simply heating Ga(NO3)3 in isopropanol at 400 °C for 24 hours, β-Ga2O3 nanoparticles with a size of ∼100 nm are obtained without requiring additional calcination in air. A structural characterization comprising X-ray diffraction, scanning electron microscopy, selected-area electron diffraction with transmission electron microscopy, and X-ray absorption fine structure measurements suggest that the synthesis process involves an initial conversion of Ga(NO3)3 to Ga(Oi-Pr)3 or a related species at 80 °C, which then transforms into γ-Ga2O3 nanoparticles upon increasing the temperature, to eventually produce β-Ga2O3 nanoparticles most likely via a dissolution and recrystallization process.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...