Rapid synthesis of Cu2O hollow spheres at low temperature and their catalytic performance for the decomposition of ammonium perchlorate†
Abstract
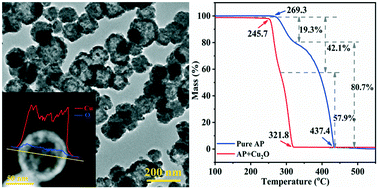
In catalytic reactions, a large specific surface area usually means more active sites. Hollow structures can provide lots of surface sites to catalyze the reaction or fix the reaction center, but the preparation process is mostly complex. In this work, using NH4+ as a structure-directing agent and ascorbic acid (AA) as a reducing agent, combined with the self-transformation process of metastable aggregated particles and the local Ostwald ripening mechanism, hollow Cu2O nanospheres with a large specific surface area (30.9 m2 g−1) were rapidly synthesized by a one-step method at low temperature. In the thermal decomposition of ammonium perchlorate (AP), it was found that the decomposition temperatures at low temperature and high temperature decreased by 31.6 °C and 125 °C, respectively. Compared with pure AP, the heat release increased by 1.5 times, and the reaction activation energy reduced by 56.0%. This is because the Cu2O hollow spheres have a larger specific surface area, and the adsorption capacity is improved owing to the loose and porous structure. The catalytic area and active sites for continuous reaction are increased, and thus the Cu2O hollow spheres exhibited excellent catalytic performance.
- This article is part of the themed collections: Nanomaterials and Crystal growth of nanomaterials


 Please wait while we load your content...
Please wait while we load your content...