Nitrochloranilic acid: a novel asymmetrically substituted quinoid bridging ligand for design of coordination polymers†
Abstract
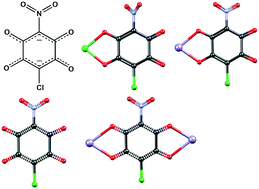
A series of alkali salts and transition metal complexes of a novel asymmetrically substituted quinoid ligand, 3-nitro-6-chloro-2,5-dihydroxyquinone (nitrochloranilic acid, H2NCA) was prepared and characterised. The nitrochloranilate moiety readily binds to transition metals either as a terminal bidentate or a bridging (bis)bidentate ligand. Therefore it is capable of forming coordination polymers of various topologies, and is a promising building block that may be used for design of metal–organic frameworks. We have prepared and structurally characterised three of its alkali salts, sodium hydrogen nitrochloranilate trihydrate (NaHNCA·3H2O), sodium nitrochloranilate trihydrate (Na2NCA·3H2O) and potassium nitrochloranilate dihydrate (K2NCA·2H2O). The first series of coordination compounds includes mononuclear {[Ni(bpy)2(NCA)]·EtOH, [Cu(phen)2(NCA)]·EtOH and (Hpy)2[Mn(H2O)2(NCA)2] (bpy = 2,2′-bipyridine, phen = 1,10-phenanthroline, Hpy = pyridinium)} and binuclear units {[Cu(bpy)(NCA)]2}, as well as 1D linear {[Cu(H2O)(MeCN)(NCA)]n}, zig-zag {[Co(phen)(NCA)]·EtOH}n and ladder-like polymers {[Mn2(NCA)2(H2O)5·3.5H2O]n}.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...