Phenoxido mediated antiferromagnetic and azide mediated ferromagnetic coupling in two dinuclear ferromagnetic nickel(ii) complexes with isomeric Schiff bases: a theoretical insight on the pathway of magnetic interaction†
Abstract
Two new dinuclear nickel(II) complexes, [(H2O)Ni(N3)(L1)(μ1,1-N3)Ni(L1)] and [(H2O)Ni(N3)(L2)(μ1,1-N3)Ni(L2)]·MeOH, derived from two isomeric Schiff base ligands, HL1 [2-{(2-(ethylamino)ethylimino)methyl}-6-ethoxyphenol] and HL2 [2-{(2-(dimethylamino)ethylimino)methyl}-6-ethoxy-phenol], have been synthesized and characterized. Variable temperature (2–300 K) magnetic susceptibility measurements indicate the presence of moderate ferromagnetic exchange coupling between nickel(II) centers. In each complex, antiferromagnetic exchange takes place through the phenoxido bridge and ferromagnetic through the μ1,1-azido bridge. The competitive interactions therefore reduce the overall magnetic coupling. In a theoretical complex, where the bridging azido ligand has been eliminated and the rest of the geometry is kept frozen, the magnetic coupling becomes antiferromagnetic which suggests that the ferromagnetic exchange occurs via the μ1,1-azido bridge. Mulliken population analysis and spin density plots clearly show that the spin distributed spherically in the Ni centers is due to the presence of one unpaired electron in both the 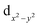 and
and 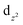 orbitals. The shape of the spin density at the bridging O-atom and azide evidences the participation of their p orbitals in the magnetic coupling. The SOMO is basically constituted by the
orbitals. The shape of the spin density at the bridging O-atom and azide evidences the participation of their p orbitals in the magnetic coupling. The SOMO is basically constituted by the 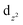 orbital of one nickel(II) center with the participation of the azide π-system. The SOMO−1 is constituted by the
orbital of one nickel(II) center with the participation of the azide π-system. The SOMO−1 is constituted by the 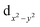 orbital of the other nickel(II), an oxygen atom and the azide π-system.
orbital of the other nickel(II), an oxygen atom and the azide π-system.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...