Effects of ligand functionalization on the band gaps and luminescent properties of a Zr12 oxo-cluster based metal–organic framework†
Abstract
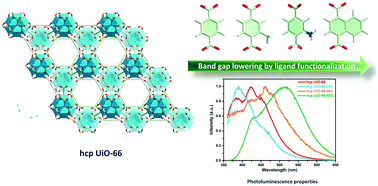
Herein, effective optical band gap engineering of a robust Zr12 oxo-based hcp UiO-66 has been realized through linker functionalization. A versatile mixed solvent-based solvothermal process was employed to synthesize the pristine hcp UiO-66 and the designed functional analogues (hcp UiO-66-CH3, hcp UiO-66-NH2 and hcp UiO-66-NDC). Optical band gaps determined from diffuse reflectance spectra (DRS) showed that hcp UiO-66 had a large band gap of 4.07 eV and a slightly narrower value of 3.93 eV was derived after the introduction of methyl groups. By embellishing the linker with an amino group possessing a strong electron-donating property, the band gap was drastically reduced to 2.92 eV. Surprisingly, the value could be further lowered to 2.48 eV by substituting the terephthalic acid (TPA) ligand of hcp UiO-66 with bulkier 1,4-naphthalic acid (1,4-NDC). In view of the tremendous influence of ligand functionalization on band gap energy, we also investigated the photoluminescence (PL) properties of the materials. It was found that the PL performance of hcp UiO-66 could also be significantly tuned by using substituted ligands: hcp UiO-66-NH2 and hcp UiO-66-NDC, which exhibited obvious red shifted PL compared to the pristine hcp UiO-66. The origins of both band gap variation and luminescence tunability were discussed accordingly.
- This article is part of the themed collection: Crystal Engineering Techniques


 Please wait while we load your content...
Please wait while we load your content...