Comparison of the effects of cation and phosphorus doping in cobalt-based spinel oxides towards the oxygen evolution reaction†
Abstract
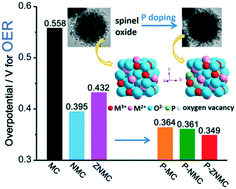
The sluggish kinetics of the oxygen evolution reaction (OER) has become a hindrance for the development of clean and sustainable energy technology and calls for highly efficient electrocatalysts. Recently, heteroatom doping has exhibited good prospects to boost the electrocatalytic performance of materials. Herein, we selected the typical MCo2O4 (M = transition metal)-type spinel oxides to test the doping effects aroused by metal cations and non-metal phosphorus (P) towards the OER in alkaline media. Not surprisingly, both cation and P dopants improved the OER activity. Additionally, P doping gave a further decrease of overpotential (reduced 34–194 mV) and Tafel slope (reduced 9.59–207.55 mV dec−1) compared to the oxide counterparts. Specifically, P-doped (Zn0.33Ni0.33Mn0.33)Co2O4 showed the best OER performance with the lowest overpotential of 0.349 V at 10 mA cm−2 and Tafel slope of 69.68 mV dec−1 and good stability after 2000 cycles of cyclic voltammetry. Redox pairs, oxygen vacancies, crystallinity and electrochemical active surface area were greatly modulated by heteroatom doping and together influenced the OER performance. This work confirms the synergistic effect of both metal and non-metal doping and the larger contribution of P dopants towards the OER, which gives a hint to develop superior electrocatalysts.
- This article is part of the themed collections: Nanomaterials and Editor’s Collection: Imperfect nanocrystals for Perfect Catalysis


 Please wait while we load your content...
Please wait while we load your content...