Ferromagnetic coupling in a dicopper(ii) oxamate complex bridged by carboxylate groups†
Abstract
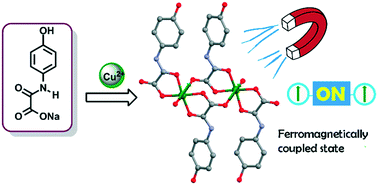
Three new air-stable coordination compounds [Cu(HL)2(H2O)2]2·2H2O (1), [Cu(HL)2]n (1a), and [Zn(HL)2(H2O)2] (2), where HL = N-(4-hydroxyphenyl)oxamate, were successfully synthesized and characterized. Single-crystal X-ray analysis reveals 1 as a dinuclear copper(II) complex, in which the metal ions are connected through the carboxylate moieties of the oxamate ligands. The crystal structure of 1a is solved using powder X-ray diffraction data and stands for an anhydrous mononuclear copper(II) oxamate complex. 2 corresponds to the first example of a mononuclear Zn2+ oxamate complex containing both ligands and water solvent molecules in the cis arrangement. The presence of a phenol group in the oxamate ligand leads to different hydrogen bond patterns in the crystal packing in 1, 1a, and 2, resulting in supramolecular 3D architectures. Magnetic measurements of 1 reveal a ferromagnetic coupling between the copper(II) ions through the carboxylate–oxamate bridge [J = +0.829 cm−1, g = 2.101, zJ = +0.154 cm−1; H = −JSCu1·SCu2, where SCu1 = SCu2 = 1/2]. DFT calculations based on the broken symmetry formalism (DFT-BS) were performed to provide insight into the magnetic behavior.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...