Experimental and theoretical evidence for oriented aggregate crystal growth of CoO in a polyol†
Abstract
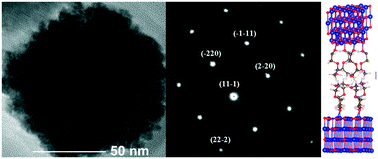
Monodispersed CoO crystals about 5 nm in size were prepared by forced hydrolysis of cobalt(II) acetate in diethyleneglycol (DEG). The adsorption of solvent molecules on these primary nanocrystals causes in situ oriented aggregation resulting in the precipitation of textured submicrometer-sized polycrystals. X-ray diffraction, infrared spectroscopy, transmission electron microscopy, thermogravimetry analysis and ab initio modelling were applied to elucidate the adsorption mechanism and the role of molecule-to-surface and molecule-to-molecule interactions in the growth mechanism of the polycrystals. We show that DEG moieties are mainly adsorbed at the top of the (100) surface cobalt atoms and that the process involves only one of the extreme oxygen atoms and not the whole molecule. As a consequence, adsorbed DEG exhibits an extended configuration which favours intermolecular hydrogen bonding from one covered nanocrystal to another. Interestingly, at high surface coverage, the energy required for DEG attachment to the crystal surface is 18.6 kJ mol−1 per molecule, while that required for hydrogen bonding between a bearing molecule and a neighbouring one is 36.4 kJ mol−1 per molecule. This means that the collective departure of a DEG assembly from the surface of CoO nanocrystals is thermodynamically easier, leading thus to the observed final morphology.
- This article is part of the themed collection: Nanomaterials


 Please wait while we load your content...
Please wait while we load your content...