Lanthanide-based molecular alloys with hydroxyterephthalate: a versatile system†
Abstract
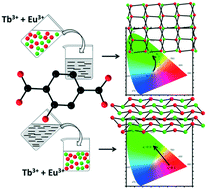
Reactions in water between lanthanide chlorides and the disodium salt of 2-hydroxyterephthalic acid (H2hbdc) lead to six families of lanthanide-based coordination polymers depending on the lanthanide ion and the crystal growth method. Compounds that constitute family F1 have the general chemical formula [Ln(Hhbdc)(hbdc)·9H2O]∞ with Ln = La–Nd and have been obtained by slow evaporation. [Ln2(hbdc)3(H2O)6·4H2O]∞ with Ln = Sm–Eu constitute family F2 and have been obtained by solvothermal synthesis. Family F3 includes compounds, obtained by a solvothermal method, with the general chemical formula [Ln2(hbdc)3(H2O)4·4H2O]∞ with Ln = Ho–Lu plus Y and compounds obtained by slow diffusion through gels with Ln = Eu–Tb. [Ln(Hhbdc)(hbdc)(H2O)3·H2O]∞ with Ln = Tb–Dy have been obtained by solvothermal methods and constitute family F4. [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5) has been obtained by slow evaporation. The last family (F6) includes compounds with the general chemical formula [Ln2(hbdc)3(H2O)8·2H2O]∞ with Ln = Nd–Tb that have been obtained by slow diffusion through gel media. Gd-Based micro-crystalline powders can be obtained by direct mixing of aqueous solutions of Gd3+ and hbdc2−. Unexpectedly, the micro-crystalline powder belongs to F5 when the lanthanide solution is added to the ligand one and to F6 when the opposite occurs. This phenomenon is also observed for the Tb- and/or Eu-based heterolanthanide coordination polymers. Their optical properties have been studied in detail.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...