Rh(iii)-catalyzed diastereoselective cascade annulation of enone-tethered cyclohexadienones via C(sp2)–H bond activation†
Abstract
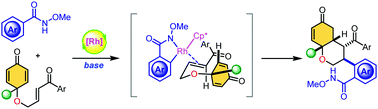
Herein, we report highly diastereoselective arylative cyclization of enone-tethered cyclohexadienones via Rh(III)-catalyzed C–H activation of N-methoxybenzamides. This reaction proceeds through the formation of a five-membered rhodacycle followed by bis-Michael cascade annulation to access functionalized bicyclic scaffolds with four contiguous stereocenters with a broad substrate scope. These products have excellent functional handles, allowing further synthetic transformation to increase the structural complexity. Furthermore, mechanistic studies of arylative cyclization and a gram-scale experiment are also presented.


 Please wait while we load your content...
Please wait while we load your content...