Cationic π-extended heteroaromatics via a catalytic C–H activation annulative alkyne-insertion sequence
Abstract
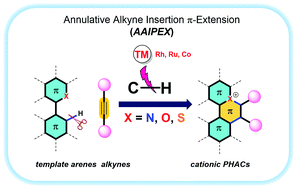
Cationic π-conjugated organic molecules have broad applications in materials science as next-generation organic materials. The annulative alkyne-insertion π-extension (AAIPEX) strategy has emerged as a promising synthetic approach for the rapid synthesis of cationic polycyclic heteroaromatic compounds (cPHACs) in a single step. The AAIPEX reaction provides a synthetic shortcut to achieve complex organic molecules from simple (hetero)arene templates and alkynes as π-extending partners, which would otherwise be difficult to achieve using traditional methods. In general, a step-economic AAIPEX protocol proceeds via C–H activation of unfunctionalized heteroarene templates, followed by alkyne insertion–annulation to furnish cPHACs. In this Feature Article, recent progress in the AAIPEX strategy to construct cPHACs is described along with brief illustrations of the resulting cPHACs in luminescence-related applications.
- This article is part of the themed collections: ChemComm Community – Dedicated Authors and Chemical Communications HOT articles


 Please wait while we load your content...
Please wait while we load your content...