Coupling of thiols and aromatic halides promoted by diboron derived super electron donors†
Abstract
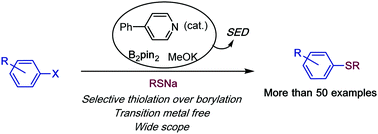
We have proven that pyridine–boryl complexes can be used as superelectron donors to promote the coupling of thiols and aromatic halides through a SRN1 mechanism. The reaction is efficient for a broad substrate scope, tolerating heterocycles including pyridines, enolizable or reducible functional groups. The method has been applied to intermediates in drug synthesis as well as interesting functionalized polythioethers through a controlled and consecutive intramolecular electron transfer process.


 Please wait while we load your content...
Please wait while we load your content...