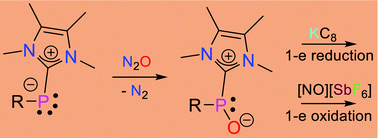
Abstract
Here we report the synthesis of an N-heterocyclic carbene (NHC)-stabilised phosphinidene oxide by the controlled oxygenation of a phosphinidene under ambient conditions. This compound can be further oxygenated to a phosphinidene dioxide. The stoichiometric reduction of a phosphinidene oxide with KC8 resembles the pinacol coupling reaction–the reduction of a carbonyl compound. We also looked at the stoichiometric oxidation of NHC-coordinated phosphinidene, phosphinidene oxide and phosphinidene dioxide with [NO][SbF6].


 Please wait while we load your content...
Please wait while we load your content...