A rearrangement of saccharin-derived cyclic ketimines with 3-chlorooxindoles leading to spiro-1,3-benzothiazine oxindoles†
Abstract
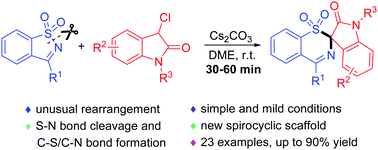
An unusual rearrangement of saccharin-derived cyclic ketimines (SDCIs) and 3-chlorooxindoles has been developed to provide a series of spiro-1,3-benzothiazine oxindoles. The reaction features simple manipulations, short reaction times, mild reaction conditions and inexpensive reagents. It is the first example where SDCIs serve as a ring-opening reagent in organic synthesis.
- This article is part of the themed collection: Chemical Communications HOT articles


 Please wait while we load your content...
Please wait while we load your content...