Atom-efficient transition-metal-free arylation of N,O-acetals using diarylzinc reagents through Zn/Zn cooperativity†
Abstract
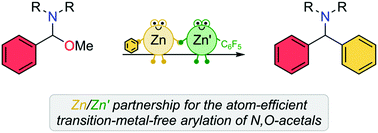
Exploiting the cooperative action of Lewis acid Zn(C6F5)2 with diarylzinc reagents, the efficient arylation of N,O-acetals to access diarylmethylamines is reported. Reactions take place under mild reaction conditions without the need for transtion-metal catalysis. Mechanistic investigations have revealed that Zn(C6F5)2 not only acts as a Lewis acid activator, but also enables the regeneration of nucleophilic ZnAr2 species, allowing a limiting 50 mol% to be employed.
- This article is part of the themed collection: ChemComm Community – Dedicated Authors


 Please wait while we load your content...
Please wait while we load your content...