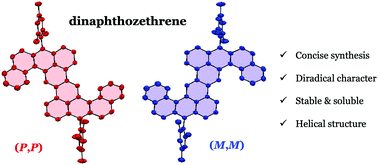
Stable and twisted 5,6:12,13-dinaphthozethrene from angular π-extension†
Abstract
Herein, we describe a concise and efficient synthesis of an angularly extended stable zethrene derivative 1, designed to have more benzenoid rings in the closed-shell resonance form. This compound exhibited enantiomeric structures in the solid state derived from the benzo[4]helicene structure and rapid interconversion in solution. Its far-red absorption, near-infrared emission and amphoteric redox properties were also revealed.


 Please wait while we load your content...
Please wait while we load your content...