Electrochemical behaviour of uranium at a tripolyphosphate modified ITO electrode†
Abstract
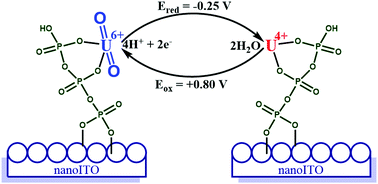
UO22+ binds to the surface of a tripolyphosphate modified mesoporous indium tin-doped oxide electrode (nanoITO|P3). Electrochemical studies reveal that nITO|P3 electrodes catalyze the 2-electron interconversion between UO22+ and U4+ with the P3-ligand assisting in the rate-limiting proton-coupled reduction of U(V) to U(IV), based on the kinetic isotope effect (1.8). Product composition between nITO|P3(U4+) and surface adsorbed UO2 can be controlled by adjusting the proton concentration and/or scan rate in voltammograms. These studies with uranium suggest that nITO|P3 electrodes are good candidates for redox transformations with other actinides including neptunium, plutonium, and americium.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...