A boron–nitrogen transborylation enabled, borane-catalysed reductive cyanation of enones†
Abstract
Cyanation offers a simple method for the introduction of a nitrile group into organic molecules and an orthogonal route for the installation of a wide array of functional groups using simple transformations. Cyanation methods are dominated by transition metal catalysis and the use of hydrogen cyanide gas. Here, the electrophilic cyanation of enones was achieved using a main-group catalyst and a non-toxic, electrophilic cyanide source. This protocol was applied across a broad substrate scope including those containing reducible functional groups. Mechanistic studies indicated an amino-borane intermediate which underwent B–N transborylation (B–N/B–H exchange) to achieve catalytic turnover.
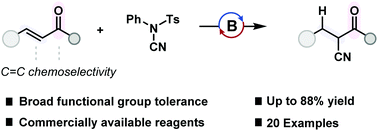
- This article is part of the themed collection: Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules


 Please wait while we load your content...
Please wait while we load your content...