Copper-catalyzed enantioselective alkene carboetherification for the synthesis of saturated six-membered cyclic ethers†
Abstract
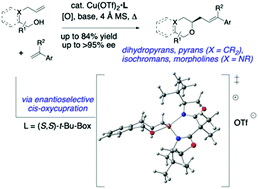
The enantioselective copper-catalyzed oxidative coupling of alkenols with styrenes for the construction of dihydropyrans, isochromans, pyrans and morpholines is reported. A concise formal synthesis of a σ1 receptor ligand using this alkene carboetherification methodology was demonstrated. Ligand, solvent and base all impact reaction efficiency. DFT transition state calculations are presented.


 Please wait while we load your content...
Please wait while we load your content...