N-Hydroxyphthalimide-catalyzed chemoselective intermolecular benzylic C–H amination of unprotected arylalkanols†
Abstract
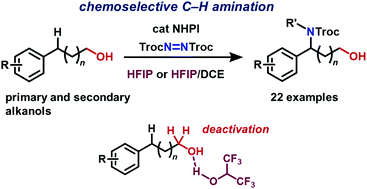
N-Hydroxyphthalimide-catalyzed chemoselective benzylic C(sp3)–H amination of unprotected arylalkanols using bis(2,2,2-trichloroethyl)azodicarboxylate has been developed. The use of 1,1,1,3,3,3-hexafluoropropan-2-ol as a solvent plays a critical role in chemoselectivity. The conversion of an aminated product to the corresponding free amino alcohol was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...