Dipolar coupling-based electron paramagnetic resonance method for protease enzymatic characterization and inhibitor screening†
Abstract
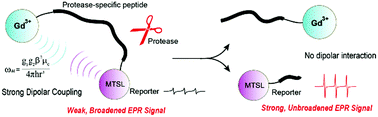
Herein, we report an EPR-based method for protease enzymatic characterization and inhibitor screening. This method utilizes dual paramagnetically-labeled probes consisting of a nitroxide spin probe and a Gd3+ ion flanking a peptide that could be specifically cleaved by protease caspase-3. Distance-dependent dipolar coupling between the two paramagnetic centers can be modulated by the protease cleavage activity, thus providing a straightforward and convenient method for protease activity detection using EPR spectroscopy under ambient conditions. Moreover, time-course monitoring of the protease-catalyzed cleavage reaction demonstrated that this EPR-based method could not only allow a direct quantitative enzymatic kinetic assessment, but also could be used for protease inhibitor screening, thus holding great potential in drug discovery studies.


 Please wait while we load your content...
Please wait while we load your content...