Enantioselective synthesis of tetra-substituted tetralines and tetrahydro-indolizines by NHC-catalyzed azolium–enolate cascade†‡
Abstract
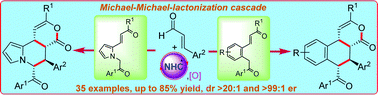
NHC-catalyzed cascade reaction of enals with suitably substituted β-(hetero)aryl enones allowing the enantioselective synthesis of tetra-substituted tetralines and tetrahydro indolizines is presented. The catalytically generated chiral α,β-unsaturated acylazoliums from enals under oxidative conditions reacted in a Michael–Michael-lactonization sequence to form the tricyclic δ-lactone products bearing four contiguous stereocentres.


 Please wait while we load your content...
Please wait while we load your content...