Design and enantioselective synthesis of 3-(α-acrylic acid) benzoxaboroles to combat carbapenemase resistance†
Abstract
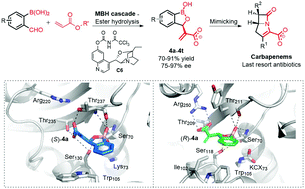
Chiral 3-substituted benzoxaboroles were designed as carbapenemase inhibitors and efficiently synthesised via asymmetric Morita–Baylis–Hillman reaction. Some of the benzoxaboroles were potent inhibitors of clinically relevant carbapenemases and restored the activity of meropenem in bacteria harbouring these enzymes. Crystallographic analyses validate the proposed mechanism of binding to carbapenemases, i.e. in a manner relating to their antibiotic substrates. The results illustrate how combining a structure-based design approach with asymmetric catalysis can efficiently lead to potent β-lactamase inhibitors and provide a starting point to develop drugs combatting carbapenemases.


 Please wait while we load your content...
Please wait while we load your content...