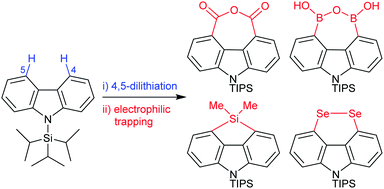
Direct formation of 4,5-disubstituted carbazoles via regioselective dilithiation†
Abstract
Carbazoles are widely exploited for their interesting photophysical and electronic properties, however bay (4,5-) functionalization is challenging, and previously inaccessible through carbazole C–H activation. We report a simple methodology which introduces a range of versatile 4,5-functionality, enabling the wider investigation of ring annulation and close proximity effects on carbazole properties.


 Please wait while we load your content...
Please wait while we load your content...