Pd-catalyzed sp–sp3 cross-coupling of benzyl bromides using lithium acetylides†
Abstract
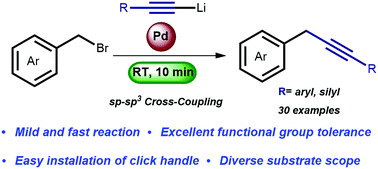
Organolithium-based cross-coupling reactions have emerged as an indispensable method to construct C–C bonds. These transformations have proven particularly useful for the direct and fast coupling of various organolithium reagents (sp, sp2, and sp3) with aromatic (pseudo) halides (sp2). Here we present an efficient method for the cross-coupling of benzyl bromides (sp3) with lithium acetylides (sp). The reaction proceeds within 10 min at room temperature and can be performed in the presence of organolithium-sensitive functional groups such as esters, nitriles, amides and boronic esters. The potential application of the methodology is demonstrated in the preparation of key intermediates used in pharmaceuticals, chemical biology and natural products.


 Please wait while we load your content...
Please wait while we load your content...