Asymmetric autocatalysis triggered by triglycine sulfate with switchable chirality by altering the direction of the applied electric field†
Abstract
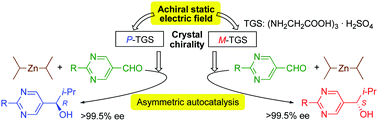
Triglycine sulfate (TGS) acts as a chiral trigger for asymmetric autocatalysis with amplification of enantiomeric excess, i.e., the Soai reaction. Therefore, molecular chirality of highly enantioenriched organic compounds is controlled by a ferroelectric crystal TGS, whose polarization is altered by an electric field.


 Please wait while we load your content...
Please wait while we load your content...