External-oxidant-free amino-benzoyloxylation of unactivated alkenes of unsaturated ketoximes with O-benzoylhydroxylamines†
Abstract
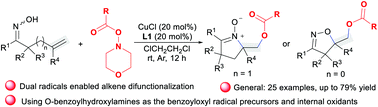
A new copper-catalyzed two-component amino-benzoyloxylation of unactivated alkenes of unsaturated ketoximes with O-benzoylhydroxylamines as the benzoyloxy sources is developed. Chemoselectivity of this method toward amino-benzoyloxylation or oxy-benzoyloxylation of alkenyl ketoximes relies on the position of the tethered olefins, and provides an external-oxidant-free alkene difunctionalization route that directly utilizes O-benzoylhydroxylamines as the benzoyloxy radical precursors and internal oxidants for the divergent synthesis of cyclic nitrones and isoxazolines.


 Please wait while we load your content...
Please wait while we load your content...