A one-pot “back-to-front” approach for the synthesis of benzene ring substituted indoles using allylboronic acids†
Abstract
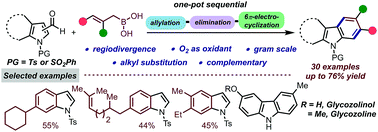
Synthesis of only benzene ring functionalized indoles and poly-substituted carbazoles is reported via a one-pot triple cascade benzannulation protocol. Usage of differently substituted and readily accessible allylboronic acids as a 3-carbon annulating partner enables diverse aliphatic and aromatic substitution patterns, which is still a daunting task. This scalable synthetic protocol tolerates broad scope, thus enabling further downstream modifications. As an application, carbazole based natural products glycozoline and glycozolinol were synthesized.


 Please wait while we load your content...
Please wait while we load your content...