Palladium catalyzed divergent cycloadditions of vinylidenecyclopropane-diesters with methyleneindolinones enabled by zwitterionic π-propargyl palladium species†
Abstract
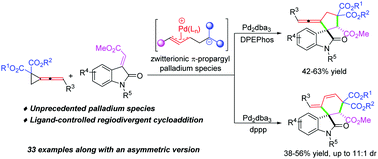
A palladium-catalyzed divergent synthesis of spirooxindoles fused with a five- or a six-membered ring by a cycloaddition reaction of vinylidenecyclopropane-diesters with methyleneindolinones was disclosed. This protocol features an in situ generated unprecedented zwitterionic π-propargyl palladium species in cycloaddition reactions and a switchable process between (3+2) and (4+2) cycloadditions by changing the phosphine ligand.
- This article is part of the themed collection: Chemical Communications HOT articles


 Please wait while we load your content...
Please wait while we load your content...