Direct synthesis of polyureas from the dehydrogenative coupling of diamines and methanol†
Abstract
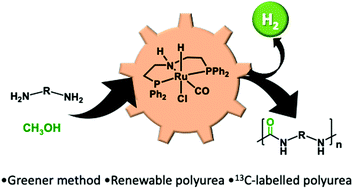
We report here the first example of the direct synthesis of polyureas from the dehydrogenative coupling of diamines and methanol using a ruthenium pincer catalyst. The present methodology replaces the use of toxic diisocyanates, conventionally used for the production of polyureas, with methanol, which is renewable, less toxic, and cheaper, making the overall process safer and more sustainable. Further advantages of the current method have been demonstrated by the synthesis of a renewable, a chiral, and the first 13C-labelled polyurea.
- This article is part of the themed collection: ChemComm Milestones – First Independent Articles


 Please wait while we load your content...
Please wait while we load your content...