Chiral recognition of amino-acid esters by a glucose-derived macrocyclic receptor†
Abstract
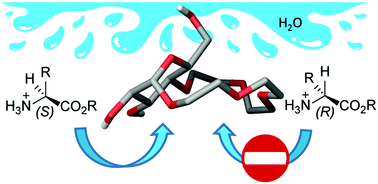
We report a glucose-based crown ether capable of chiral recognition of a wide range of amino-acid methyl esters in aqueous environments. The enantioselectivities towards amino-acids with extended hydrophobic side chains displayed by the glucose-derived macrocycle are among the highest observed for small molecule synthetic receptors to date.
- This article is part of the themed collection: ChemComm Milestones – First Independent Articles


 Please wait while we load your content...
Please wait while we load your content...