Selective demethylation of O-aryl glycosides by iridium-catalyzed hydrosilylation†
Abstract
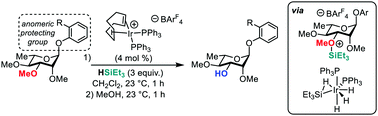
The cleavage of alkyl ethers by hydrosilylation is a powerful synthetic tool for the generation of silyl ethers. Previous attempts to apply this transformation to carbohydrate derivatives have been constrained by poor selectivity and preferential reduction of the anomeric position. O-Aryl glycosides are found to be stable under iridium- and borane-catalyzed hydrosilylation conditions, allowing for alkyl ether cleavage without loss of anomeric functionality. A cationic bis(phosphine)iridium complex catalyzes the selective 3-demethylation of a variety of 2,3,4-tri-O-methyl pyranoses, offering a unique approach to 3-hydroxy or 3-acetyl 2,4-di-O-methylpyranoses.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...