Semisynthetic head-to-tail cyclized peptides obtained by combining protein trans-splicing and intramolecular expressed protein ligation†
Abstract
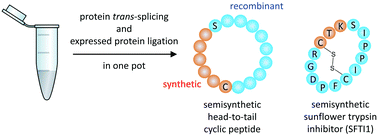
A dual-intein approach for the preparation of head-to-tail macrocyclic peptides is reported, where synthetic and genetically encoded fragments are ligated by two native peptide bonds. A split intein ligates the synthetic and genetically encoded peptides via protein trans-splicing and is followed by intramolecular cyclization through an expressed protein ligation step mediated with a cis-intein. We identified a suitable pair of orthogonal inteins and optimized the conditions for a one-pot cyclization protocol. We report the semisynthesis of model macrocyles with various ring sizes and of the natural product sunflower trypsin inhibitor (SFTI) along with its ornithine analog.


 Please wait while we load your content...
Please wait while we load your content...