Oxidative cross-coupling processes inspired by the Chan–Lam reaction
Abstract
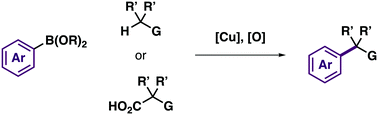
The Cu-catalyzed oxidative cross-coupling of N- and O-nucleophiles with aryl boronic acids (the Chan–Lam reaction) remains among the most useful approaches to prepare aniline and phenol derivatives. The combination of high chemoselectivity, mild reaction conditions, and the ability to use simple Cu-salts as catalysts makes this process a valuable alternative to aromatic substitutions and Pd-catalyzed reactions of aryl electrophiles (Buchwald–Hartwig coupling). Despite the widespread use of Chan–Lam reactions in synthesis, the analogous carbon–carbon bond forming variant of this process had not been developed prior to our work. This feature article describes our discovery and application of Cu-catalyzed oxidative coupling reactions of activated methylene derivatives or carboxylic acids with nucleophiles including aryl boronic esters and amines.


 Please wait while we load your content...
Please wait while we load your content...