Enantioselective preparation of atropisomeric biaryl trifluoromethylsulfanes via ring-opening of cyclic diaryliodoniums†
Abstract
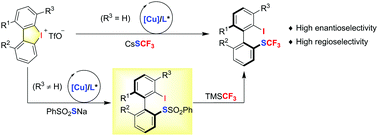
Two convenient and practical methods for the synthesis of axially chiral biaryls bearing the trifluoromethylthio group are reported. A Cu-catalyzed enantioselective ring-opening reaction of cyclic diaryliodoniums with CsSCF3 enables the direct synthesis of trifluoromethylthiolated biaryl atropisomers in high yields and enantioselectivity. For unsymmetric cyclic diaryliodoniums bearing an adjacent group to the C–I bond, a two-step procedure is required to achieve good regio- and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...