Diverse diaryl sulfide synthesis through consecutive aryne reactions†
Abstract
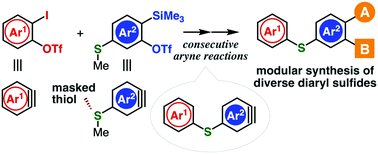
An efficient method to synthesize diaryl sulfides with structural diversity is disclosed. Demethylative hydrothiolation of aryne intermediates generated from o-iodoaryl triflates with methylthio-substituted o-silylaryl triflates and further aryne reactions afford diverse diaryl sulfides.


 Please wait while we load your content...
Please wait while we load your content...