Ion mobility-mass spectrometry shows stepwise protein unfolding under alkaline conditions†
Abstract
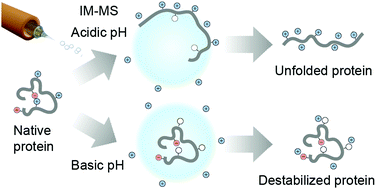
Although native mass spectrometry is widely applied to monitor chemical or thermal protein denaturation, it is not clear to what extent it can inform about alkali-induced unfolding. Here, we probe the relationship between solution- and gas-phase structures of proteins under alkaline conditions. Native ion mobility-mass spectrometry reveals that globular proteins are destabilized rather than globally unfolded, which is supported by solution studies, providing detailed insights into alkali-induced unfolding events. Our results pave the way for new applications of MS to monitor structures and interactions of proteins at high pH.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...