Ribosome-mediated incorporation of fluorescent amino acids into peptides in vitro†
Abstract
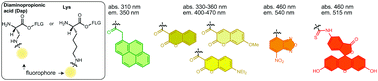
We report the design, chemical synthesis, and flexizyme-catalyzed transfer RNA (tRNA) acylation of a variety of fluorescent amino acids (FAAs). The fluorescent groups include pyrene, coumarin, nitrobenzoxadiazole, and fluorescein variants. We further demonstrate site-specific incorporation of the FAAs into peptides by the ribosome in vitro through genetic code reprogramming.


 Please wait while we load your content...
Please wait while we load your content...