Molecular evolution of a cytochrome P450 for the synthesis of potential antidepressant (2R,6R)-hydroxynorketamine†
Abstract
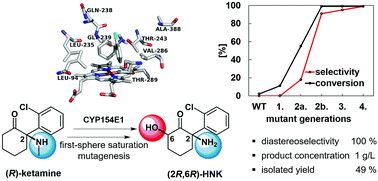
Saturation mutagenesis at seven first-sphere residues of the cytochrome P450 monooxygenase 154E1 (CYP154E1) from Thermobifida fusca YX was applied to construct a variant with only three substitutions that enabled the effective two-step synthesis of the potential antidepressant (2R,6R)-hydroxynorketamine. A recombinant E. coli whole-cell system was essential for GC/MS based medium-throughput screening and at the same time facilitated the oxidation of the substrate (R)-ketamine at a higher scale for product isolation and subsequent NMR analysis.


 Please wait while we load your content...
Please wait while we load your content...