Untangling the interaction of α-synuclein with DNA i-motifs and hairpins by volume-sensitive single-molecule FRET spectroscopy†
Abstract
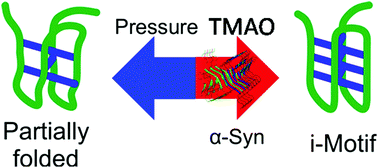
The intrinsically disordered protein α-synuclein causes Parkinson's disease by forming toxic oligomeric aggregates inside neurons. Single-molecule FRET experiments revealed conformational changes of noncanonical DNA structures, such as i-motifs and hairpins, in the presence of α-synuclein. Volumetric analyses revealed differences in binding mode, which is also affected by cellular osmolytes.


 Please wait while we load your content...
Please wait while we load your content...