The evaluation of the chemical quality and UV overall components dissolution consistency of Flos Chrysanthemi Indici preparation†
Abstract
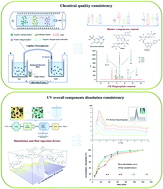
In recent years, new chemical technology and chemical analysis methods have been widely used for the quality control of medicine to provide security for human life. However, for the increasingly popular traditional Chinese medicine (TCM) preparations, the traditional quality control technology has been challenged due the presence of multi-components. To solve this problem, this study proposed a comprehensive evaluation strategy from two aspects: chemical quality and in vitro dissolution consistency. Zhenju antihypertensive tablet (FCIP), as the research object of this study, is a compound preparation of Flos Chrysanthemi Indici with moderate antihypertensive effect. For the chemical quality, the capillary electrophoresis fingerprint (CE-FP) was established based on the characteristic multi-wavelength averaging fusion profiling (CMW-AFP) strategy, which can fuse peaks in the CE chromatogram at several select characteristic wavelengths into one profile through an averaging algorithm. All samples were clarified into 5 quality grades using the systematic quantified fingerprint method, and showed significant difference among the four manufacturers. Combined with the accurate determination of the marker components, the CE-FP evaluation results can provide a guarantee for the chemical quality consistency. For the in vitro dissolution consistency, a UV overall components dissolution method was proposed. Meanwhile, in order to match the established UV overall dissolution system, two f2 factors (f2-R and f2-MI) were calculated to compare the dissolution profiles. By comparing the chemical and UV overall dissolution results plus the PCA analysis, the holistic quality of the FCIP samples of four manufacturers were obtained as MA > MC > MD > MB. The established evaluation system is also a suitable strategy for controlling the chemical quality and dissolution consistency of other TCM preparations.
- This article is part of the themed collection: Analytical Methods HOT Articles 2021


 Please wait while we load your content...
Please wait while we load your content...