Determination of atomoxetine levels in human plasma using LC-MS/MS and clinical application to Chinese children with ADHD based on CPIC guidelines
Abstract
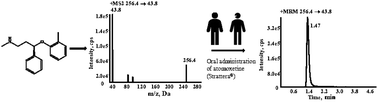
The Clinical Pharmacogenetic Implementation Consortium (CPIC) guidelines for personalized atomoxetine therapy are based on the CYP2D6 genotype information and the peak plasma concentrations of atomoxetine. Therefore, a highly rapid, sensitive, and reproducible method is critical for the clinical implementation of the guidelines. In this study, an LC-MS/MS approach was developed and validated for the determination of atomoxetine levels in human plasma using atomoxetine-d3 as the internal standard. Samples were prepared by simple protein precipitation method with MeOH. The analyte was separated using a Kinetex C18 column (2.1 mm × 50 mm, 2.6 μm, Phenomenex) with a flow rate of 0.25 mL min−1, using a gradient elution. A MeOH and water solution containing 5 mM ammonium acetate and 0.1 mM formic acid (pH 6.26) was used as the mobile phase and successfully solved the problem of inconsistent retention time between the plasma samples and the solution samples of atomoxetine. Detection was performed under positive-electrospray-ion multiple reaction-monitoring mode using the 256.4 → 43.8 and 259.3 → 47.0 transitions for atomoxetine and atomoxetine-d3, respectively. Linearity was achieved using an extremely wide range, from 0.500 to 2000 ng mL−1 in plasma. The intra- and inter-batch precision and accuracy, dilution accuracy, recovery, and stability of the method were all within the acceptable limits and no matrix effect was observed. With a complex needle wash solution containing ACN : MeOH : isopropanol : H2O (4 : 4:1 : 1, v/v/v/v), carryover contamination was eliminated successfully. This method was successfully implemented on pediatric patients with attention-deficit/hyperactivity disorder and provided valuable information to enable clinicians to do dose selection and titration.


 Please wait while we load your content...
Please wait while we load your content...