Influence of protein ion charge state on 213 nm top-down UVPD†
Abstract
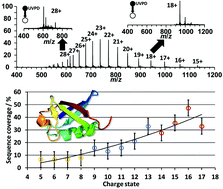
Ultraviolet photodissociation (UVPD) is a powerful and rapidly developing method in top-down proteomics. Sequence coverages can exceed those obtained with collision- and electron-induced fragmentation methods. Because of the recent interest in UVPD, factors that influence protein fragmentation and sequence coverage are actively debated in the literature. Here, we performed top-down 213 nm UVPD experiments on a 7 T Fourier-transform ion cyclotron resonance mass spectrometer (FT-ICR MS) for the model proteins ubiquitin, myoglobin and cytochrome c that were electrosprayed from native, denaturing and supercharging solutions in order to investigate the effect of protein charge states on UVPD fragments. By performing UVPD in ultrahigh vacuum, factors associated with collisional cooling and any ion activation during transfer between mass analyzers can be largely eliminated. Sequence coverage increased from <10% for low charge states to >60% for high charge states for all three proteins. This trend is influenced by the overall charge state, i.e., charges per number of amino acid residues, and to a lesser degree by associated structural changes of protein ions of different charge states based on comparisons to published collision-cross section measurements. To rationalize this finding, and correlate sequence ion formation and identity with the number and location of protons, UVPD results were compared to protonation sites predicted based on electrostatic modelling. Assuming confined protonation sites, these results indicate the presence of two general fragmentation types; i.e., charge remote and charge directed. For moderately high protein charge states, fragment ions mostly originate in regions between likely protonation sites (charge remote), whereas sequence ions of highly charge protein ions occur either near backbone amide protonation sites at low-basicity residues (charge directed) or at charge remote sites (i.e., high-basicity residues). Overall, our results suggest that top-down 213 UVPD performance in the zero-pressure limit depends strongly on protein charge states and protonation sites can influence the location of backbone cleavages.
- This article is part of the themed collection: 150th Anniversary Collection: Mass Spectrometry


 Please wait while we load your content...
Please wait while we load your content...