Enhanced detection sensitivity of the chemisorption of pyridine and biotinylated proteins at localized surface plasmon resonance inflection points in single gold nanorods†
Abstract
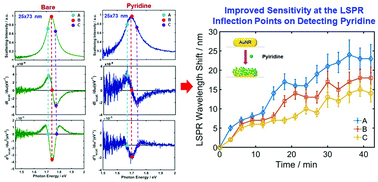
Plasmonic gold nanoparticles have been widely used for localized surface plasmon resonance (LSPR) sensing. Herein, we investigate the enhanced sensitivity for the detection of the chemisorption of pyridine and biotinylated bovine serum albumin (BSA) proteins, which are important molecules widely used in biological studies, at the inflection points (IFs) of the LSPR scattering spectra of single gold nanorods (AuNRs). The results showed that the homogeneous LSPR IFs located at the long wavelength side (or low energy side) of the LSPR scattering peak exhibited the highest sensitivity for the detection of chemical adsorption with respect to the counterpart LSPR peak maxima. The increased sensitivity can be attributed to the shape change of the LSPR peak when the local refractive index is increased by chemisorption. Furthermore, real-time monitoring of molecular binding events on single AuNRs was performed after introducing pyridine in water, and an improved efficiency of the sensors was observed at the LSPR IFs to detect target molecules in single AuNRs. Therefore, we present the significance of tracking curvature shapes through homogeneous LSPR IFs close to the resonance energy upon chemical adsorption of pyridine and BSA-biotin, rather than tracking their counterpart LSPR maximum peak shifts, for AuNRs.


 Please wait while we load your content...
Please wait while we load your content...