Asymmetric N-heteroacene tetracene analogues as potential n-type semiconductors†
Abstract
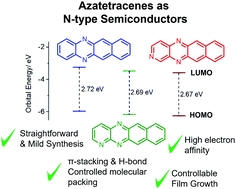
In the search for high performance n-type organic semiconductors (OSCs) a simple strategy might be substitution of aromatic CH groups for nitrogen heteroatoms. Here, we report the synthesis and characterisation of two novel N-heteroacene compounds, namely, 1,5,12-triazatetracene (TrAT1) and 2,5,12-triazatetracene (TrAT2). Their potential as n-type materials is evaluated against 5,12-diazatetracene (DAT) by UV/vis and EPR spectroscopy, cyclic voltammetry, DFT, single crystal X-ray diffraction and thin film characterisation. Increasing the number of N-heteroatoms was found to stabilise the HOMO and LUMO leading to electron affinities for TrAT1 and TrAT2 of ca. −4 eV. Both compounds were found to exhibit columns of co-facial π-stacked molecules. For TrAT1, molecules are also linked by hydrogen bonding, while the crystal structure of TrAT2 was found to be inherently disordered. Thin films of DAT, TrAT1 and TrAT2 were grown by organic molecular beam deposition (OMBD) and found to form discontinuous films, where TrAT1 exhibited a preferential orientation.


 Please wait while we load your content...
Please wait while we load your content...